Abstract
Objectives
A continuous intervention based on healthcare management agreements was associated in our hospital with an increase in the absolute number of spontaneous reporting of adverse drug reactions (ADRs), and also with an increase in the number of reports of serious or unexpected ADRs and ADRs associated with new drugs. The objective was to analyse the effect of this intervention on the features of ADRs spontaneously reported in a hospital, the drugs involved and the number of signals identified.
Methods
A longitudinal study with two periods, the 1st period without intervention from 1998 to 2002 and the 2nd period with intervention from 2003 to 2005, was carried out in a tertiary teaching hospital. Changes between the two periods with regard to the following variables were analysed: the patients’ characteristics, such as gender and age; the reported ADRs, and the medical assistance required; the suspected drugs involved in the ADRs; the main signals identified.
Results
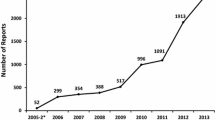
Gender and age distribution of patients described in the spontaneous reports were no different in the two periods. During the second period, spontaneously reported cases requiring hospital admission and those occurring in hospital increased (236 from 2 in the first period and 277 from 99 in the first period respectively) and cases from outpatient hospital consultations began to be reported (13.9% of reports). The spontaneous reporting on all kinds of ADRs and drugs increased during the second period. Cutaneous reactions were the most frequently spontaneously reported ADRs in both periods followed by cardiovascular and neurological reactions in the first period, and haematological and gastrointestinal reactions in the second one. However, during the second period the higher increase was for endocrinological, urinary and hepatic reactions. Systemic antibiotics, anti-thrombotics and cardiac therapy drugs were the most common therapeutic subgroups reported to be suspected drugs in both periods, but in the second period the proportion of immunostimulants, beta blocking agents, immunosuppressants and psychoanaleptics increased. No signals were recognised during the first period; however, two signals and one additional safety concern were identified during the second.
Conclusion
An intervention based on healthcare management agreements, was associated with an important increase in spontaneous reporting of ADRs by hospital physicians and also with a change in terms of the type of ADRs identified affecting different organs or systems, and the therapeutic groups of drugs involved. Future studies should analyse the effect of different types of intervention on the spontaneous reporting of ADRs in hospitals.
Similar content being viewed by others
References
Wysowski DK, Swartz L (2005) Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med 165:1363–1369
Aronson JK (2007) Adverse drug reactions—no farewell to harms. Br J Clin Pharmacol 63:131–135
Thürmann PA (2001) Methods and systems to detect adverse drug reactions in hospitals. Drug Saf 24:961–968
Rawlins MD (1995) Pharmacovigilance; paradise lost, regained or postponed? The William Withering Lecture 1994. J R Coll Physicians Lond 29:41–49
Vallano A, Cereza G, Pedros C, Agusti A, Danes I, Aguilera C, Arnau JM (2005) Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol 60:653–658
Moride Y, Haramburu F, Requejo AA, Begaud B (1997) Under-reporting of adverse drug reactions in general practice. Br J Clin Pharmacol 43:177–181
Figueiras A, Herdeiro MT, Polonia J, Gestal-Otero JJ (2006) An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA 296:1086–1093
Nazario M, Feliu JF, Rivera GC (1994) Adverse drug reactions: the San Juan Department of Veterans Affairs Medical Center experience. Hosp Pharm 29:244–250
Scott HD, Thacher-Renshaw A, Rosenbaum SE, Waters WJ Jr, Green M, Andrews LG, Faich GA (1990) Physician reporting of adverse drug reactions. Results of the Rhode Island adverse drug reaction reporting project. JAMA 263:1785–1788
Fincham J (1989) A statewide program to stimulate reporting of adverse drug reactions. J Pharm Pract 2:239–244
McGettigan P, Golden J, Conroy RM, Arthur N, Feely J (1997) Reporting of adverse drug reactions by hospital doctors and the response to intervention. Br J Clin Pharmacol 44:98–100
Kimelblatt BJ, Young SH, Heywood PM, Mandala AR, Gendelman S, Mehl B (1988) Improved reporting of adverse drug reactions. Am J Hosp Pharm 45:1086–1089
Pedrós C, Vallano A, Cereza G, Mendoza-Aran G, Agustí A, Aguilera C, Danés I, Vidal X, Arnau JM (2009) An intervention to improve spontaneous adverse drug reaction reporting by hospital physicians. A time series analysis in Spain. Drug Saf 32:77–83
Maistrello I, Morgutti M, Maltempi M, Dantes M (1995) Adverse drug reactions in hospitalized patients: an operational procedure to improve reporting and investigate underreporting. Pharmacoepidemiol Drug Saf 4:101–106
Cox AR, Anton C, Goh CHF, Easter M, Langford NJ, Ferner RE (2001) Adverse drug reactions in patients admitted to hospital identified by discharge ICD-10 codes and by spontaneous reports. Br J Clin Pharmacol 52:337–339
Ortega A, Aguinagalde A, Lacasa C, Aquerreta I, Fernández-Benítez M, Fernández LM (2008) Efficacy of an adverse drug reaction electronic reporting system integrated into a hospital information system. Ann Pharmacother 42:1491–1496
Jose J, Rao PGM (2006) Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharmacol Res 54:226–233
Arulmani R, Rajendran SD, Suresh B (2007) Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol 65:210–216
Baniasadi S, Fahimi F, Shalviri G (2008) Developing an adverse drug reaction reporting system at a teaching hospital. Basic Clin Pharmacol Toxicol 102:408–411
Edwards R (1997) Adverse drug reactions: finding the needle in the haystack. Br Med J 315:500
Commission Directive 2000/38/EC of 5 June 2000 amending Chapter Va (Pharmacovigilance) of Council Directive 75/319/EEC on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products. Official Journal of the European Communities 10.6.2000: L139/28-L139/30. Available from http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:139:0028:0030:EN:PDF. Accessed 10 September 2009
Capellà D, Laporte J-R (1993) La notificación espontánea de reacciones adversas a medicamentos. In: Laporte J-R, Tognoni G (eds) Principios de epidemiología del medicamento, 2nd edn. Masson-Salvat, Barcelona, pp 147–170
Spanish Agency of Medicines and Health Products. Product Information. Gelafundina®. https://sinaem4.agemed.es/consaem/especialidad.do?metodo=verFichaWordPdf&codigo=61627&formato=pdf&formulario=FICHAS. Accessed 10 September 2009
Spanish Agency of Medicines and Health Products. Informative note. Recommendations for the withdrawal of the treatment with Agreal® (Veralipride). http://www.agemed.es/actividad/alertas/docs/NI_2005-15_AGREAL.pdf. Accessed 10 September 2009
European Medicines Agency. Questions and answers on the recommendation to withdraw the marketing authorisation of veralipride. http://www.emea.europa.eu/pdfs/human/referral/agreal/29946807en.pdf. Accessed 10 September 2009
European Medicines Agency. Xigris. Procedural steps taken and scientific information after the authorisation. Changes made after 01/10/2004. http://www.emea.europa.eu/humandocs/PDFs/EPAR/xigris/H-396-en8b.pdf. Accessed 10 September 2009
Uppal R, Jhaj R, Malhotra S (2000) Adverse drug reactions among inpatients in a north Indian referral hospital. Natl Med J India 13:16–18
Lugardon S, Desboeuf K, Fernet P, Monstastruc J-L, Lapeyre-Mestre M (2006) Using a capture-recapture method to assess the frequency of adverse drug reactions in a French university hospital. Br J Clin Pharmacol 62:225–231
Acknowledgements
The authors would like to thank the hospital physicians who participated in this study for their collaboration. No sources of funding were used to assist in the development of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cereza, G., Agustí, A., Pedrós, C. et al. Effect of an intervention on the features of adverse drug reactions spontaneously reported in a hospital. Eur J Clin Pharmacol 66, 937–945 (2010). https://doi.org/10.1007/s00228-010-0856-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0856-8